Which Teory of Matter is Belived Today Discontinuous or Continuous
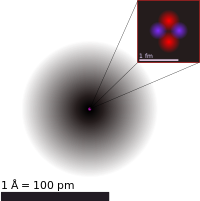
The current theoretical model of the atom involves a dense nucleus surrounded by a probabilistic "cloud" of electrons
Atomic theory is the scientific theory that matter is composed of particles called atoms. Atomic theory traces its origins to an ancient philosophical tradition known as atomism. According to this idea, if one were to take a lump of matter and cut it into ever smaller pieces, one would eventually reach a point where the pieces could not be further cut into anything smaller. Ancient Greek philosophers called these hypothetical ultimate particles of matter atomos, a word which meant "uncut".
In the early 1800s, the scientist John Dalton noticed that chemical substances seemed to combine and break down into other substances by weight in proportions that suggested that each chemical element is ultimately made up of tiny indivisible particles of consistent weight. Shortly after 1850, certain physicists developed the kinetic theory of gases and of heat, which mathematically modelled the behavior of gases by assuming that they were made of particles. In the early 20th century, Albert Einstein and Jean Perrin proved that Brownian motion (the erratic motion of pollen grains in water) is caused by the action of water molecules; this third line of evidence silenced remaining doubts among scientists as to whether atoms and molecules were real. Throughout the nineteenth century, some scientists had cautioned that the evidence for atoms was indirect, and therefore atoms might not actually be real, but only seem to be real.
By the early 20th century, scientists had developed fairly detailed and precise models for the structure of matter, which led to more rigorously-defined classifications for the tiny invisible particles that make up ordinary matter. An atom is now defined as the basic particle that composes a chemical element. Around the turn of the 20th century, physicists discovered that the particles that chemists called "atoms" are in fact agglomerations of even smaller particles (subatomic particles), but scientists kept the name out of convention. The term elementary particle is now used to refer to particles that are actually indivisible.
History
Philosophical atomism
The idea that matter is made up of discrete units is a very old idea, appearing in many ancient cultures such as Greece and India. The word "atom" (Greek: ἄτομος ; atomos ), meaning "uncuttable", was coined by the Pre-Socratic Greek philosophers Leucippus and his pupil Democritus (c.460–c.370 BC).[1] [2] [3] [4] Democritus taught that atoms were infinite in number, uncreated, and eternal, and that the qualities of an object result from the kind of atoms that compose it.[2] [3] [4] Democritus's atomism was refined and elaborated by the later Greek philosopher Epicurus (341–270 BC), and by the Roman Epicurean poet Lucretius (c.99–c.55 BC).[3] [4] During the Early Middle Ages, atomism was mostly forgotten in western Europe. During the 12th century, it became known again in western Europe through references to it in the newly-rediscovered writings of Aristotle.[3] The opposing view of matter upheld by Aristotle was that matter was continuous and infinite and could be subdivided without limit.[5] [6]
In the 14th century, the rediscovery of major works describing atomist teachings, including Lucretius's De rerum natura and Diogenes Laërtius's Lives and Opinions of Eminent Philosophers, led to increased scholarly attention on the subject. Nonetheless, because atomism was associated with the philosophy of Epicureanism, which contradicted orthodox Christian teachings, belief in atoms was not considered acceptable by most European philosophers.[3] The French Catholic priest Pierre Gassendi (1592–1655) revived Epicurean atomism with modifications, arguing that atoms were created by God and, though extremely numerous, are not infinite. He was the first person who used the term "molecule" to describe aggregation of atoms.[3] [4] Gassendi's modified theory of atoms was popularized in France by the physician François Bernier (1620–1688) and in England by the natural philosopher Walter Charleton (1619–1707). The chemist Robert Boyle (1627–1691) and the physicist Isaac Newton (1642–1727) both defended atomism and, by the end of the 17th century, it had become accepted by portions of the scientific community.[3]
John Dalton

Near the end of the 18th century, two laws about chemical reactions emerged without referring to the notion of an atomic theory. The first was the law of conservation of mass, closely associated with the work of Antoine Lavoisier, which states that the total mass in a chemical reaction remains constant (that is, the reactants have the same mass as the products).[7] The second was the law of definite proportions. First established by the French chemist Joseph Proust in 1797 this law states that if a compound is broken down into its constituent chemical elements, then the masses of the constituents will always have the same proportions by weight, regardless of the quantity or source of the original substance.[8]
John Dalton studied and expanded upon this previous work and defended a new idea, later known as the law of multiple proportions: if the same two elements can be combined to form a number of different compounds, then the ratios of the masses of the two elements in their various compounds will be represented by small whole numbers. This is a common pattern in chemical reactions that was observed by Dalton and other chemists at the time.
Example 1 — tin oxides: Dalton identified two oxides of tin. One is a grey powder in which for every 100 parts of tin there is 13.5 parts of oxygen. The other oxide is a white powder in which for every 100 parts of tin there is 27 parts of oxygen.[9] 13.5 and 27 form a ratio of 1:2. These oxides are today known as tin(II) oxide (SnO) and tin(IV) oxide (SnO2) respectively.
Example 2 — iron oxides: Dalton identified two oxides of iron. One is a black powder in which for every 100 parts of iron there is about 28 parts of oxygen. The other is a red powder in which for every 100 parts of iron there is 42 parts of oxygen.[10] 28 and 42 form a ratio of 2:3. These oxides are today known as iron(II) oxide (better known as wüstite) and iron(III) oxide (the major constituent of rust). Their formulas are FeO and Fe2O3 respectively.
Example 3 — nitrogen oxides: There are three oxides of nitrogen in which for every 140 g of nitrogen, there is 80 g, 160 g, and 320 g of oxygen respectively, which gives a ratio of 1:2:4. These are nitrous oxide (N2O), nitric oxide (NO), and nitrogen dioxide (NO2) respectively.
This recurring pattern suggested that chemicals do not react in any arbitrary quantity, but in multiples of some basic indivisible unit of mass.
In his writings, Dalton used the term "atom" to refer to the basic particle of any chemical substance, not strictly for elements as is the practice today. Dalton did not use the word "molecule"; instead, he used the terms "compound atom" and "elementary atom".[11] Dalton proposed that each chemical element is composed of atoms of a single, unique type, and though they cannot be altered or destroyed by chemical means, they can combine to form more complex structures (chemical compounds). This marked the first truly scientific theory of the atom, since Dalton reached his conclusions by experimentation and examination of the results in an empirical fashion.
In 1803 Dalton referred to a list of relative atomic weights for a number of substances in a talk before the Manchester Literary and Philosophical Society on the solubility of various gases, such as carbon dioxide and nitrogen, in water. Dalton did not indicate how he obtained the relative weights, but he initially hypothesized that variation in solubility was due to differences in mass and complexity of the gas particles – an idea that he abandoned by the time the paper was finally published in 1805.[12] Over the years, several historians have attributed the development of Dalton's atomic theory to his study of gaseous solubility, but a recent study of his laboratory notebook entries concludes he developed the chemical atomic theory in 1803 to reconcile Cavendish's and Lavoisier's analytical data on the composition of nitric acid, not to explain the solubility of gases in water.[13]
Thomas Thomson published the first brief account of Dalton's atomic theory in the third edition of his book, A System of Chemistry.[14] In 1808 Dalton published a fuller account in the first part of A New System of Chemical Philosophy.[15] However, it was not until 1811 that Dalton provided his rationale for his theory of multiple proportions.[16]
Dalton estimated the atomic weights according to the mass ratios in which they combined, with the hydrogen atom taken as unity. However, Dalton did not conceive that with some elements atoms exist in molecules—e.g. pure oxygen exists as O2. He also mistakenly believed that the simplest compound between any two elements is always one atom of each (so he thought water was HO, not H2O).[17] This, in addition to the crudity of his equipment, flawed his results. For instance, in 1803 he believed that oxygen atoms were 5.5 times heavier than hydrogen atoms, because in water he measured 5.5 grams of oxygen for every 1 gram of hydrogen and believed the formula for water was HO. Adopting better data, in 1806 he concluded that the atomic weight of oxygen must actually be 7 rather than 5.5, and he retained this weight for the rest of his life. Others at this time had already concluded that the oxygen atom must weigh 8 relative to hydrogen equals 1, if one assumes Dalton's formula for the water molecule (HO), or 16 if one assumes the modern water formula (H2O).[18]
Avogadro
The flaw in Dalton's theory was corrected in principle in 1811 by Amedeo Avogadro. Avogadro had proposed that equal volumes of any two gases, at equal temperature and pressure, contain equal numbers of molecules (in other words, the mass of a gas's particles does not affect the volume that it occupies).[19] Avogadro's law allowed him to deduce the diatomic nature of numerous gases by studying the volumes at which they reacted. For instance: since two liters of hydrogen will react with just one liter of oxygen to produce two liters of water vapor (at constant pressure and temperature), it meant a single oxygen molecule splits in two in order to form two particles of water. Thus, Avogadro was able to offer more accurate estimates of the atomic mass of oxygen and various other elements, and made a clear distinction between molecules and atoms.
Brownian Motion
In 1827, the British botanist Robert Brown observed that dust particles inside pollen grains floating in water constantly jiggled about for no apparent reason. In 1905, Albert Einstein theorized that this Brownian motion was caused by the water molecules continuously knocking the grains about, and developed a hypothetical mathematical model to describe it.[20] This model was validated experimentally in 1908 by French physicist Jean Perrin, thus providing additional validation for particle theory (and by extension atomic theory).
Statistical Mechanics
In order to introduce the Ideal gas law and statistical forms of physics, it was necessary to postulate the existence of atoms. In 1738, Swiss physicist and mathematician Daniel Bernoulli postulated that the pressure of gases and heat were both caused by the underlying motion of molecules.
In 1860, James Clerk Maxwell, who was a vocal proponent of atomism, was the first to use statistical mechanics in physics.[21] Ludwig Boltzmann and Rudolf Clausius expanded his work on gases and the laws of Thermodynamics especially the second law relating to entropy. In the 1870s, Josiah Willard Gibbs, sometimes referred to as America's greatest physicist,[22] extended the laws of entropy and thermodynamics and coined the term "statistical mechanics." Einstein later independently reinvented Gibb's laws, because they had only been printed in an obscure American journal.[23] Einstein later commented, had he known of Gibb's work he would "not have published those papers at all, but confined myself to the treatment of some few points [that were distinct]."[24] All of statistical mechanics and the laws of heat, gas, and entropy were necessarily postulated upon the existence of atoms.
Discovery of subatomic particles


The cathode rays (blue) were emitted from the cathode, sharpened to a beam by the slits, then deflected as they passed between the two electrified plates.
Atoms were thought to be the smallest possible division of matter until 1897 when J. J. Thomson discovered the electron through his work on cathode rays.[25]
A Crookes tube is a sealed glass container in which two electrodes are separated by a vacuum. When a voltage is applied across the electrodes, cathode rays are generated, creating a glowing patch where they strike the glass at the opposite end of the tube. Through experimentation, Thomson discovered that the rays could be deflected by an electric field (in addition to magnetic fields, which was already known). He concluded that these rays, rather than being a form of light, were composed of very light negatively charged particles he called "corpuscles" (they would later be renamed electrons by other scientists). He measured the mass-to-charge ratio and discovered it was 1800 times smaller than that of hydrogen, the smallest atom. These corpuscles were a particle unlike any other previously known.
Thomson suggested that atoms were divisible, and that the corpuscles were their building blocks.[26] To explain the overall neutral charge of the atom, he proposed that the corpuscles were distributed in a uniform sea of positive charge; this was the plum pudding model[27] as the electrons were embedded in the positive charge like raisins in a plum pudding (although in Thomson's model they were not stationary). The reason J. J. Thomson's spherical positive charge model interspersed with negative electrons was most widely accepted over several different versions of nuclear planetary models was that the Thompson model could best align with classical physics. Solar system models proposed before Thompson always resulted in electrons spiraling into the nucleus.[28]
Discovery of the nucleus
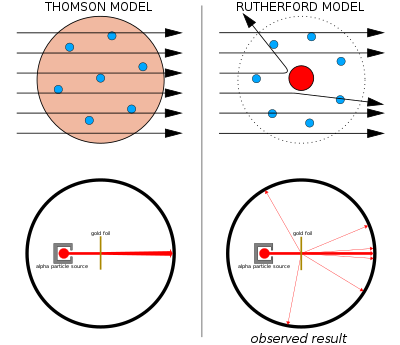
The Geiger–Marsden experiment
Left: Expected results: alpha particles passing through the plum pudding model of the atom with negligible deflection.
Right: Observed results: a small portion of the particles were deflected by the concentrated positive charge of the nucleus.
Thomson's plum pudding model was disproved in 1909 by one of his former students, Ernest Rutherford, who discovered that most of the mass and positive charge of an atom is concentrated in a very small fraction of its volume, which he assumed to be at the very center.
Ernest Rutherford and his colleagues Hans Geiger and Ernest Marsden came to have doubts about the Thomson model after they encountered difficulties when they tried to build an instrument to measure the charge-to-mass ratio of alpha particles (these are positively-charged particles emitted by certain radioactive substances such as radium). The alpha particles were being scattered by the air in the detection chamber, which made the measurements unreliable. Thomson had encountered a similar problem in his work on cathode rays, which he solved by creating a near-perfect vacuum in his instruments. Rutherford didn't think he'd run into this same problem because alpha particles are much heavier than electrons. According to Thomson's model of the atom, the positive charge in the atom is not concentrated enough to produce an electric field strong enough to deflect an alpha particle, and the electrons are so lightweight they should be pushed aside effortlessly by the much heavier alpha particles. Yet there was scattering, so Rutherford and his colleagues decided to investigate this scattering carefully.[29]
Between 1908 and 1913, Rutherford and his colleagues performed a series of experiments in which they bombarded thin foils of metal with alpha particles. They spotted alpha particles being deflected by angles greater than 90°. To explain this, Rutherford proposed that the positive charge of the atom is not distributed throughout the atom's volume as Thomson believed, but is concentrated in a tiny nucleus at the center. Only such an intense concentration of charge could produce an electric field strong enough to deflect the alpha particles as observed.[29] Rutherford's model is sometimes called the "planetary model".[30] However, Hantaro Nagaoka was quoted by Rutherford as the first to suggest a planetary atom in 1904.[31] And planetary models had been suggested as early as 1897 such as the one by Joseph Larmor.[32] Probably the earliest solar system model was found in an unpublished note by Ludwig August Colding in 1854 whose idea was that atoms were analogous to planetary systems that rotate and cause magnetic polarity.[33]
First steps toward a quantum physical model of the atom
The planetary model of the atom had two significant shortcomings. The first is that, unlike planets orbiting a sun, electrons are charged particles. An accelerating electric charge is known to emit electromagnetic waves according to the Larmor formula in classical electromagnetism. An orbiting charge should steadily lose energy and spiral toward the nucleus, colliding with it in a small fraction of a second. The second problem was that the planetary model could not explain the highly peaked emission and absorption spectra of atoms that were observed.

Quantum theory revolutionized physics at the beginning of the 20th century, when Max Planck and Albert Einstein postulated that light energy is emitted or absorbed in discrete amounts known as quanta (singular, quantum). This led to a series of quantum atomic models such as the quantum model of Arthur Erich Haas in 1910 and the 1912 John William Nicholson quantum atomic model that quantized angular momentum as h/2π.[34] [35] In 1913, Niels Bohr incorporated this idea into his Bohr model of the atom, in which an electron could only orbit the nucleus in particular circular orbits with fixed angular momentum and energy, its distance from the nucleus (i.e., their radii) being proportional to its energy.[36] Under this model an electron could not spiral into the nucleus because it could not lose energy in a continuous manner; instead, it could only make instantaneous "quantum leaps" between the fixed energy levels.[36] When this occurred, light was emitted or absorbed at a frequency proportional to the change in energy (hence the absorption and emission of light in discrete spectra).[36]
Bohr's model was not perfect. It could only predict the spectral lines of hydrogen; it couldn't predict those of multielectron atoms. Worse still, as spectrographic technology improved, additional spectral lines in hydrogen were observed which Bohr's model couldn't explain. In 1916, Arnold Sommerfeld added elliptical orbits to the Bohr model to explain the extra emission lines, but this made the model very difficult to use, and it still couldn't explain more complex atoms.
Discovery of isotopes
While experimenting with the products of radioactive decay, in 1913 radiochemist Frederick Soddy discovered that there appeared to be more than one element at each position on the periodic table.[37] The term isotope was coined by Margaret Todd as a suitable name for these elements.
That same year, J. J. Thomson conducted an experiment in which he channeled a stream of neon ions through magnetic and electric fields, striking a photographic plate at the other end. He observed two glowing patches on the plate, which suggested two different deflection trajectories. Thomson concluded this was because some of the neon ions had a different mass.[38] The nature of this differing mass would later be explained by the discovery of neutrons in 1932.
Discovery of nuclear particles
In 1917 Rutherford bombarded nitrogen gas with alpha particles and observed hydrogen nuclei being emitted from the gas (Rutherford recognized these, because he had previously obtained them bombarding hydrogen with alpha particles, and observing hydrogen nuclei in the products). Rutherford concluded that the hydrogen nuclei emerged from the nuclei of the nitrogen atoms themselves (in effect, he had split a nitrogen).[39]
From his own work and the work of his students Bohr and Henry Moseley, Rutherford knew that the positive charge of any atom could always be equated to that of an integer number of hydrogen nuclei. This, coupled with the atomic mass of many elements being roughly equivalent to an integer number of hydrogen atoms - then assumed to be the lightest particles - led him to conclude that hydrogen nuclei were singular particles and a basic constituent of all atomic nuclei. He named such particles protons. Further experimentation by Rutherford found that the nuclear mass of most atoms exceeded that of the protons it possessed; he speculated that this surplus mass was composed of previously-unknown neutrally charged particles, which were tentatively dubbed "neutrons".
In 1928, Walter Bothe observed that beryllium emitted a highly penetrating, electrically neutral radiation when bombarded with alpha particles. It was later discovered that this radiation could knock hydrogen atoms out of paraffin wax. Initially it was thought to be high-energy gamma radiation, since gamma radiation had a similar effect on electrons in metals, but James Chadwick found that the ionization effect was too strong for it to be due to electromagnetic radiation, so long as energy and momentum were conserved in the interaction. In 1932, Chadwick exposed various elements, such as hydrogen and nitrogen, to the mysterious "beryllium radiation", and by measuring the energies of the recoiling charged particles, he deduced that the radiation was actually composed of electrically neutral particles which could not be massless like the gamma ray, but instead were required to have a mass similar to that of a proton. Chadwick now claimed these particles as Rutherford's neutrons.[40] For his discovery of the neutron, Chadwick received the Nobel Prize in 1935.
Quantum physical models of the atom

The five filled atomic orbitals of a neon atom separated and arranged in order of increasing energy from left to right, with the last three orbitals being equal in energy. Each orbital holds up to two electrons, which most probably exist in the zones represented by the colored bubbles. Each electron is equally present in both orbital zones, shown here by color only to highlight the different wave phase.
In 1924, Louis de Broglie proposed that all moving particles—particularly subatomic particles such as electrons—exhibit a degree of wave-like behavior. Erwin Schrödinger, fascinated by this idea, explored whether or not the movement of an electron in an atom could be better explained as a wave rather than as a particle. Schrödinger's equation, published in 1926,[41] describes an electron as a wave function instead of as a point particle. This approach elegantly predicted many of the spectral phenomena that Bohr's model failed to explain. Although this concept was mathematically convenient, it was difficult to visualize, and faced opposition.[42] One of its critics, Max Born, proposed instead that Schrödinger's wave function did not describe the physical extent of an electron (like a charge distribution in classical electromagnetism), but rather gave the probability that an electron would, when measured, be found at a particular point.[43] This reconciled the ideas of wave-like and particle-like electrons: the behavior of an electron, or of any other subatomic entity, has both wave-like and particle-like aspects, and whether one aspect or the other is more apparent depends upon the situation.[44]
A consequence of describing electrons as waveforms is that it is mathematically impossible to simultaneously derive the position and momentum of an electron. This became known as the Heisenberg uncertainty principle after the theoretical physicist Werner Heisenberg, who first published a version of it in 1927.[45] (Heisenberg analyzed a thought experiment where one attempts to measure an electron's position and momentum simultaneously. However, Heisenberg did not give precise mathematical definitions of what the "uncertainty" in these measurements meant. The precise mathematical statement of the position-momentum uncertainty principle is due to Earle Hesse Kennard, Wolfgang Pauli, and Hermann Weyl.[46] [47]) This invalidated Bohr's model, with its neat, clearly defined circular orbits. The modern model of the atom describes the positions of electrons in an atom in terms of probabilities. An electron can potentially be found at any distance from the nucleus, but, depending on its energy level and angular momentum, exists more frequently in certain regions around the nucleus than others; this pattern is referred to as its atomic orbital. The orbitals come in a variety of shapes—sphere, dumbbell, torus, etc.—with the nucleus in the middle.[48] The shapes of atomic orbitals are found by solving the Schrödinger equation; however, analytic solutions of the Schrödinger equation are known for very few relatively simple model Hamiltonians including the hydrogen atom and the dihydrogen cation. Even the helium atom—which contains just two electrons—has defied all attempts at a fully analytic treatment.
See also
- Spectroscopy
- History of molecular theory
- Timeline of chemical element discoveries
- Introduction to quantum mechanics
- Kinetic theory of gases
- Atomism
- The Physical Principles of the Quantum Theory
Footnotes
- ^ Pullman, Bernard (1998). The Atom in the History of Human Thought. Oxford, England: Oxford University Press. pp. 31–33. ISBN978-0-19-515040-7.
- ^ a b Kenny, Anthony (2004). Ancient Philosophy. A New History of Western Philosophy. Vol. 1. Oxford, England: Oxford University Press. pp. 26–28. ISBN0-19-875273-3.
- ^ a b c d e f g Pyle, Andrew (2010). "Atoms and Atomism". In Grafton, Anthony; Most, Glenn W.; Settis, Salvatore (eds.). The Classical Tradition. Cambridge, Massachusetts and London, England: The Belknap Press of Harvard University Press. pp. 103–104. ISBN978-0-674-03572-0.
- ^ a b c d Cohen, Henri; Lefebvre, Claire, eds. (2017). Handbook of Categorization in Cognitive Science (Second ed.). Amsterdam, The Netherlands: Elsevier. p. 427. ISBN978-0-08-101107-2.
- ^ "Welcome to CK-12 Foundation | CK-12 Foundation".
- ^ Berryman, Sylvia, "Democritus", The Stanford Encyclopedia of Philosophy (Fall 2008 Edition), Edward N. Zalta (ed.), http://plato.stanford.edu/archives/fall2008/entries/democritus
- ^ Weisstein, Eric W. "Lavoisier, Antoine (1743-1794)". scienceworld.wolfram.com. Retrieved 2009-08-01 .
- ^ "Law of definite proportions | chemistry". Encyclopedia Britannica . Retrieved 2020-09-03 .
- ^ Dalton (1817). A New System of Chemical Philosophy vol. 2, p. 36
- ^ Dalton (1817). A New System of Chemical Philosophy vol. 2, p. 28
- ^ Dalton (1817). A New System of Chemical Philosophy vol. 2, p. 281
- ^ Dalton, John. "On the Absorption of Gases by Water and Other Liquids", in Memoirs of the Literary and Philosophical Society of Manchester. 1803. Retrieved on August 29, 2007.
- ^ Grossman, Mark I. (2021-01-02). "John Dalton's "Aha" Moment: the Origin of the Chemical Atomic Theory". Ambix. 68 (1): 49–71. doi:10.1080/00026980.2020.1868861. ISSN 0002-6980. PMID 33577439. S2CID 231909410.
- ^ "Thomas Thomson on Dalton's Atomic Hypothesis". www.chemteam.info . Retrieved 2021-02-20 .
- ^ Dalton, John (1808). A New System of Chemical Philosophy ... S. Russell. pp. 211–216.
- ^ Nicholson, William (1811). A Journal of Natural Philosophy, Chemistry and the Arts. G. G. and J. Robinson. pp. 143–151.
- ^ Johnson, Chris. "Avogadro - his contribution to chemistry". Archived from the original on 2002-07-10. Retrieved 2009-08-01 .
- ^ Alan J. Rocke (1984). Chemical Atomism in the Nineteenth Century. Columbus: Ohio State University Press.
- ^ Avogadro, Amedeo (1811). "Essay on a Manner of Determining the Relative Masses of the Elementary Molecules of Bodies, and the Proportions in Which They Enter into These Compounds". Journal de Physique. 73: 58–76.
- ^ Einstein, A. (1905). "Über die von der molekularkinetischen Theorie der Wärme geforderte Bewegung von in ruhenden Flüssigkeiten suspendierten Teilchen" (PDF). Annalen der Physik. 322 (8): 549–560. Bibcode:1905AnP...322..549E. doi:10.1002/andp.19053220806. hdl:10915/2785.
- ^ See:
- Maxwell, J.C. (1860) "Illustrations of the dynamical theory of gases. Part I. On the motions and collisions of perfectly elastic spheres," Philosophical Magazine, 4th series, 19 : 19–32.
- Maxwell, J.C. (1860) "Illustrations of the dynamical theory of gases. Part II. On the process of diffusion of two or more kinds of moving particles among one another," Philosophical Magazine, 4th series, 20 : 21–37.
- ^ See Wikipedia article on Gibbs
- ^ Navarro, Luis. "Gibbs, Einstein and the Foundations of Statistical Mechanics." Archive for History of Exact Sciences, vol. 53, no. 2, Springer, 1998, pp. 147–80, http://www.jstor.org/stable/41134058.
- ^ Stone, A. Douglas, Einstein and the quantum : the quest of the valiant Swabian, Princeton University Press, (2013). ISBN 978-0-691-13968-5 quoted from Folsing, Albert Einstein, 110.
- ^ Thomson, J. J. (1897). "Cathode rays" ([facsimile from Stephen Wright, Classical Scientific Papers, Physics (Mills and Boon, 1964)]). Philosophical Magazine. 44 (269): 293. doi:10.1080/14786449708621070.
- ^ Whittaker, E. T. (1951), A History of the Theories of Aether and Electricity. Vol 1, Nelson, London
- ^ Thomson, J. J. (1904). "On the Structure of the Atom: an Investigation of the Stability and Periods of Oscillation of a number of Corpuscles arranged at equal intervals around the Circumference of a Circle; with Application of the Results to the Theory of Atomic Structure". Philosophical Magazine. 7 (39): 237. doi:10.1080/14786440409463107.
- ^ Kumar, Manjit, Quantum Einstein and Bohr Great Debate, Icon Books, 2009
- ^ a b Heilbron (2003). Ernest Rutherford and the Explosion of Atoms, pp. 64-68
- ^ "Rutherford model | Definition & Facts". Encyclopedia Britannica . Retrieved 23 August 2021.
- ^ Rutherford either knew the article or looked it up, for he cited it on the last page of his classic paper, "The Scattering of a and b Particles by Matter and the Structure of the Atom," Phil. Mag., 21 (1911), 669.
- ^ Larmor, Joseph (1897), , Philosophical Transactions of the Royal Society, 190: 205–300, Bibcode:1897RSPTA.190..205L, doi:10.1098/rsta.1897.0020 "…that of the transmission of radiation across a medium permeated by molecules, each consisting of a system of electrons in steady orbital motion, and each capable of free oscillations about the steady state of motion with definite free periods analogous to those of the planetary inequalities of the Solar System;"
- ^ Helge Kragh, Niels Bohr and the Quantum Atom: The Bohr Model of Atomic Structure 1913–1925, 2012, Chap. 1, ISBN 9780199654987, Oxford Scholarship Online, doi:10.1093/acprof:oso/9780199654987.001.0001
- ^ J. W. Nicholson, Month. Not. Roy. Astr. Soc. lxxii. pp. 49,130, 677, 693, 729 (1912).
- ^ The Atomic Theory of John William Nicholson, Russell McCormmach, Archive for History of Exact Sciences, Vol. 3, No. 2 (25.8.1966), pp. 160-184 (25 pages), Springer.
- ^ a b c Bohr, Niels (1913). "On the constitution of atoms and molecules" (PDF). Philosophical Magazine. 26 (153): 476–502. Bibcode:1913PMag...26..476B. doi:10.1080/14786441308634993. Archived (PDF) from the original on 2022-10-09.
- ^ "Frederick Soddy, The Nobel Prize in Chemistry 1921". Nobel Foundation. Retrieved 2008-01-18 .
- ^ Thomson, J. J. (1913). "Rays of positive electricity". Proceedings of the Royal Society. A 89 (607): 1–20. Bibcode:1913RSPSA..89....1T. doi:10.1098/rspa.1913.0057. [as excerpted in Henry A. Boorse & Lloyd Motz, The World of the Atom, Vol. 1 (New York: Basic Books, 1966)]. Retrieved on August 29, 2007.
- ^ Rutherford, Ernest (1919). "Collisions of alpha Particles with Light Atoms. IV. An Anomalous Effect in Nitrogen". Philosophical Magazine. 37 (222): 581. doi:10.1080/14786440608635919.
- ^ Chadwick, James (1932). "Possible Existence of a Neutron" (PDF). Nature. 129 (3252): 312. Bibcode:1932Natur.129Q.312C. doi:10.1038/129312a0. S2CID 4076465. Archived (PDF) from the original on 2022-10-09.
- ^ Schrödinger, Erwin (1926). "Quantisation as an Eigenvalue Problem". Annalen der Physik. 81 (18): 109–139. Bibcode:1926AnP...386..109S. doi:10.1002/andp.19263861802.
- ^ Mahanti, Subodh. "Erwin Schrödinger: The Founder of Quantum Wave Mechanics". Archived from the original on 2009-04-17. Retrieved 2009-08-01 .
- ^ Mahanti, Subodh. "Max Born: Founder of Lattice Dynamics". Archived from the original on 2009-01-22. Retrieved 2009-08-01 .
- ^ Greiner, Walter (4 October 2000). "Quantum Mechanics: An Introduction". ISBN9783540674580 . Retrieved 2010-06-14 .
- ^ Heisenberg, W. (1927). "Über den anschaulichen Inhalt der quantentheoretischen Kinematik und Mechanik". Zeitschrift für Physik (in German). 43 (3–4): 172–198. Bibcode:1927ZPhy...43..172H. doi:10.1007/BF01397280. S2CID 122763326.
- ^ Busch, Paul; Lahti, Pekka; Werner, Reinhard F. (17 October 2013). "Proof of Heisenberg's Error-Disturbance Relation". Physical Review Letters. 111 (16): 160405. arXiv:1306.1565. Bibcode:2013PhRvL.111p0405B. doi:10.1103/PhysRevLett.111.160405. ISSN 0031-9007. PMID 24182239. S2CID 24507489.
- ^ Appleby, David Marcus (6 May 2016). "Quantum Errors and Disturbances: Response to Busch, Lahti and Werner". Entropy. 18 (5): 174. arXiv:1602.09002. Bibcode:2016Entrp..18..174A. doi:10.3390/e18050174.
- ^ Milton Orchin; Roger Macomber; Allan Pinhas; R. Wilson. "The Vocabulary and Concepts of Organic Chemistry, Second Edition" (PDF). Archived (PDF) from the original on 2022-10-09. Retrieved 2010-06-14 .
Bibliography
- Andrew G. van Melsen (1960) [First published 1952]. From Atomos to Atom: The History of the Concept Atom. Translated by Henry J. Koren. Dover Publications. ISBN0-486-49584-1.
- J. P. Millington (1906). John Dalton. J. M. Dent & Co. (London); E. P. Dutton & Co. (New York).
- Jaume Navarro (2012). A History of the Electron: J. J. and G. P. Thomson. Cambridge University Press. ISBN978-1-107-00522-8.
Further reading
- Bernard Pullman (1998) The Atom in the History of Human Thought, trans. by Axel Reisinger. Oxford Univ. Press.
- Eric Scerri (2007) The Periodic Table, Its Story and Its Significance, Oxford University Press, New York.
- Charles Adolphe Wurtz (1881) The Atomic Theory, D. Appleton and Company, New York.
- Alan J. Rocke (1984) Chemical Atomism in the Nineteenth Century: From Dalton to Cannizzaro, Ohio State University Press, Columbus (open access full text at http://digital.case.edu/islandora/object/ksl%3Ax633gj985).
External links
- Atomism by S. Mark Cohen.
- Atomic Theory - detailed information on atomic theory with respect to electrons and electricity.
- The Feynman Lectures on Physics Vol. I Ch. 1: Atoms in Motion
Source: https://en.wikipedia.org/wiki/Atomic_theory
0 Response to "Which Teory of Matter is Belived Today Discontinuous or Continuous"
Post a Comment